Research Area-1
5-lipoxygenase (5-LO), 5-lipoxygenase-activating protein (FLAP) and microsomal prostaglandin E2 synthase-1 (mPGES-1) are important targets for treatment of chronic inflammatory and cardiovascular diseases and cancer [1-3]. There are no drugs approved for any of these targets except for Zileuton, an iron chelating 5-LO inhibitor, which has a very limited use due to pharmacokinetic and toxicity problems [4]. Therefore, our goal is the discovery, development and evaluation of appropriate molecular pharmacological inhibitors of 5-LO, FLAP and/or the mPGES-1. Synthesis, computer-aided processing of compounds and evaluation of their mechanisms of action at the molecular and cellular level take place in close cooperation with external collaborators. An important aspect of our research activities include the combined use of chemical, computational and pharmacological tools in order to eventually provide new chemotypes for novel therapies.
Inhibition of leukotriene biosynthesis targeting FLAP
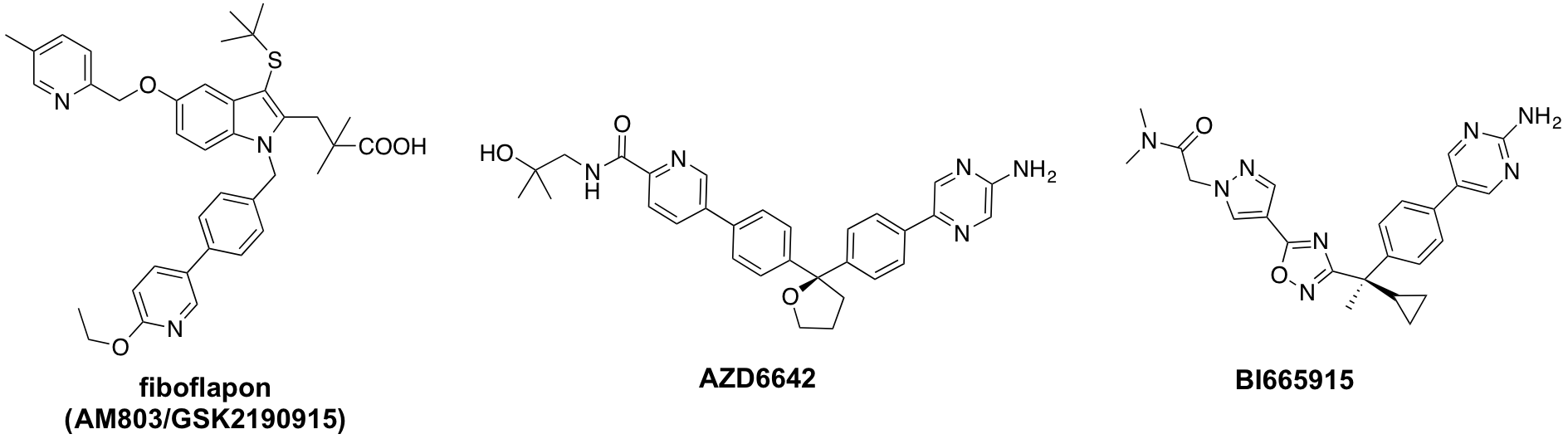
5-lipoxygenase-activating protein (FLAP) is an 18-kDa integral membrane protein which is essential for cellular leukotriene (LT) synthesis, and is the target of LT biosynthesis inhibitors. During the last two-decades, there has been growing interest in the development of FLAP inhibitors from pharmaceutical industry. These efforts have resulted in several FLAP inhibitors such as fiboflapon (GSK2190915, 3), AZD6642 (4) and BI665915 (5), which are reported to be in various phases of preclinical and clinical studies for treatment of respiratory diseases such as asthma and COPD [5-7]. In addition, beneficial pharmacological outcomes in atherosclerosis by inhibition of LT biosynthesis was demonstrated, indicating the therapeutic potential of this target for cardiovascular diseases besides respiratory pathologies [8].
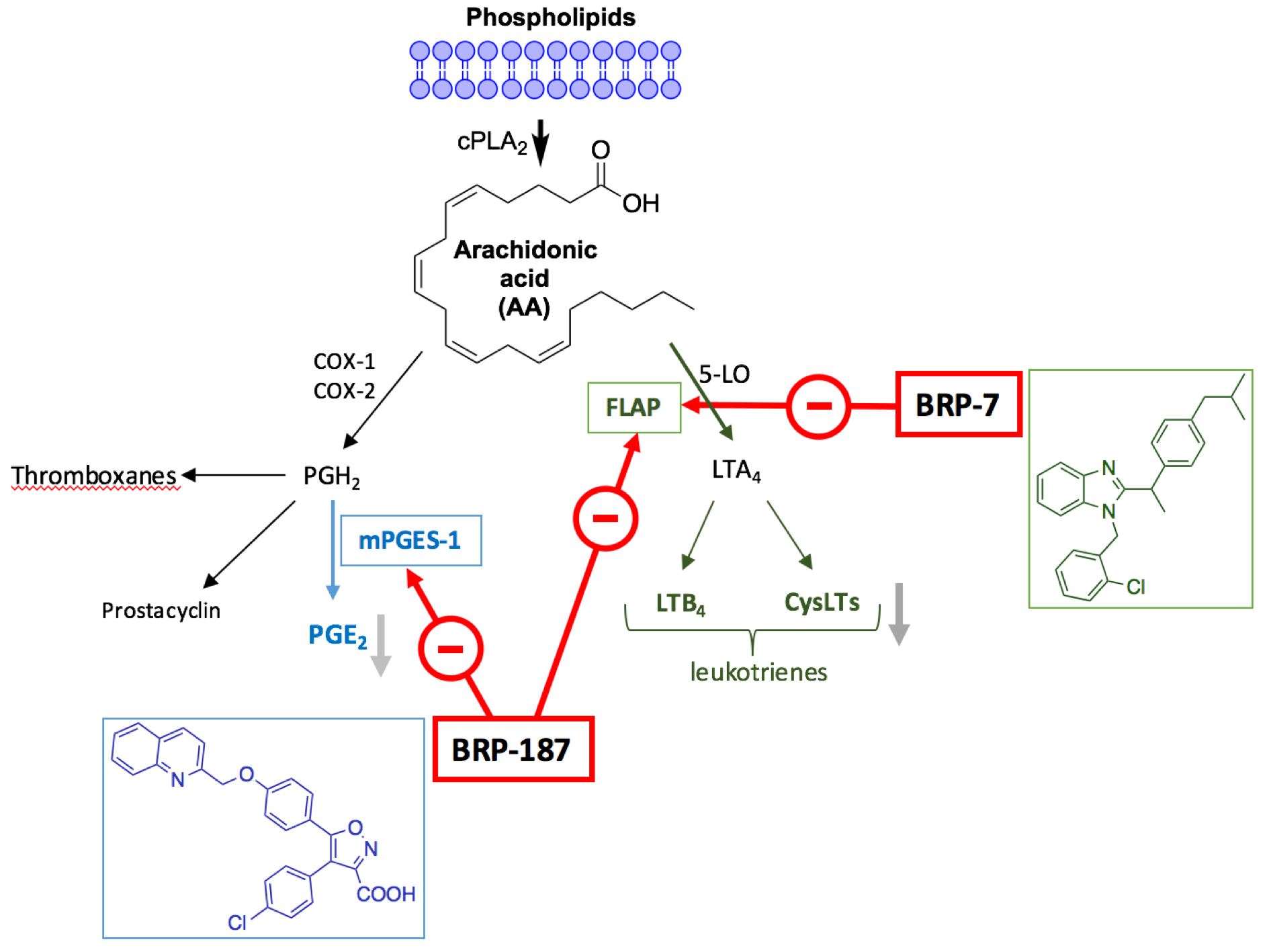
Along this line of research, our group recently identified a non-acidic benzimidazole derivative (BRP-7) bearing an ibuprofen fingerprint with reasonable potency to suppress 5-LO product synthesis in intact neutrophils (IC50 = 0.31 μM) [9]. Our detailed pharmacological investigation revealed BRP-7 as a potent inhibitor of LT biosynthesis, selectively targeting FLAP without affecting other related enzymes in the AA cascade (i.e., 5-LO, COXs, mPGES-1) [10]. We also introduced a second chemotype, an isoxazole derivative BRP-187 for inhibition of LT biosynthesis targeting FLAP [11]. This compound elicits its activity by preventing the 5-LO/FLAP interaction at the nuclear envelope of human neutrophils. BRP-187 also potently inhibited microsomal prostaglandin E2 synthase-1 (mPGES-1) thereby representing a new chemical class, which is developable for dual mPGES-1/FLAP inhibitors. Microsomal PGE synthase-1 (mPGES-1): New therapeutic target for next generation anti-inflammatory drugs PGE2 is a lipid mediator serving pivotal roles in pathologies with inflammation, fewer and pain. mPGES-1 is an inducible terminal enzyme in the production of PGE2 and solely responsible for PGE2 synthesis during inflammation. Therefore, it is the critical enzyme for inflammation, pain, angiogenesis, tumorigenesis, and atherosclerosis, and presents an attractive target to achieve more specific inhibition of PGE2 production in disease states while preserving synthesis of other PGs and, as such, has attracted considerable attention as a promising target for “next-generation” anti-inflammatory drugs [2].
Research Area-2
New therapeutic approaches for treatment of cancer have been of interest for both academic researchers and drug companies, and the number of studies on new anticancer drug development continues to grow quickly. Akt pathway presents an exciting new target for molecular therapeutics, as it functions as a cardinal nodal point for transducing extracellular (growth factor and insulin) and intracellular (receptor tyrosine kinases, Ras and Src) oncogenic signals. In addition, alterations of the Akt pathway have been detected in a number of human malignancies. Ectopic expression of Akt, especially constitutively activated Akt, is sufficient to induce oncogenic transformation of cells and tumor formation in transgenic mice. Blockage of Akt signaling results in apoptosis and growth inhibition of tumor cells with elevated Akt. The observed dependence of certain tumors on Akt signaling for survival and growth has wide implications for cancer therapy, including breast and liver cancer [12]. Based on our preliminary studies fundamental to our ongoing research activities, different chemotypes from in-house combinatorial library were tested against liver cancer cell lines. We have discovered a lead compound BRP-445 with potent antiproliferative activity, especially showing selectivity against liver cancer cell lines such as HEPG2, HEP3B, MAHLAVU, FOCUS and SNU475. We further demonstrated that the BRP-445 caused apoptosis based on the cell cycle arrest at SubG1/G1 phase, inhibition of Akt protein phosphorylation and also by causing increased amounts of cleaved PARP in Mahlavu cell lines. In parallel to the in vitro results, in vivo efficacy of BRP-445 was demonstrated by its inhibition of tumor volume (50%) in xenografts established with Mahlavu cell lines.
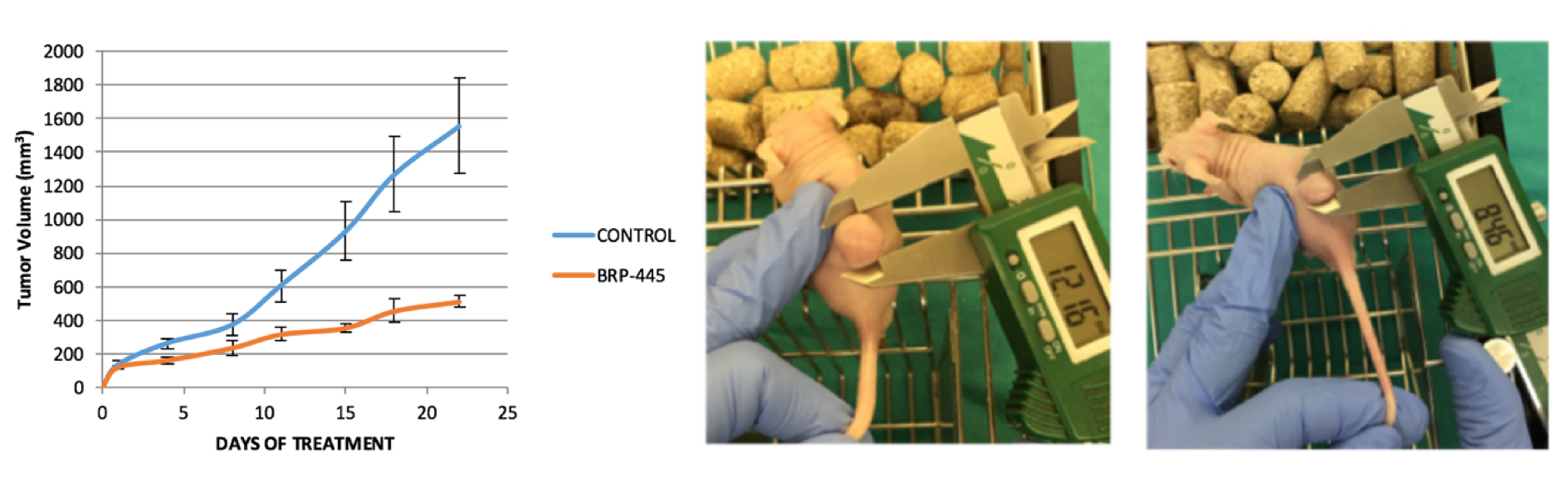
Within the context of these results, our group has been engaged in lead compound optimization towards in vitro/in vivo preclinical studies with the aim of developing and patenting novel drug candidates targeting Akt pathway. The main goal of the project is therefore to obtain candidate compounds with optimized drug-like properties, which will be active in in vivo athymic nude mice xenograft tumor models. Projects Studies on the Development of 5-Lipoxygenase (5-LO) and 5-Lipoxygenase activating protein (FLAP) Inhibitors (TÜBİTAK 108S210) Development of 5-Lipoxygenase activating protein (FLAP) Inhibitors (TÜBİTAK 112S596) Investigations on the Synthesis, Structure-Activity Relationships and Mechanisms of Action of Novel Pyrazole Derivatives with Antiplatelet Activity (TÜBİTAK 110S106) Investigations on the Synthesis, Structure-Activity Relationships and Mechanisms of Action of Novel Isoxazole Derivatives with Potential Anticancer Activity (TÜBİTAK 214S062) Development and Preclinical Studies of New Drug Candidates Targeting PI3K/Akt Signaling Pathway for Treatment of Breast and Liver Cancer (TÜBİTAK 215S015) Synthesis of [6-(3,5-Dimethylpyrazol-1-yl)-3(2H)-pyridazinon-2-yl]acetamide Derivatives and their in vitro COX-2 Inhibitory Activities (TÜBİTAK 2631)
References
[1] B. Hofmann, D. Steinhilber, 5-Lipoxygenase inhibitors: a review of recent patents (2010-2012), Expert Opin. Ther. Pat., 23 (2013) 895-909. [2] A. Koeberle, O. Werz, Perspective of microsomal prostaglandin E2 synthase-1 as drug target in inflammation-related disorders, Biochem. Pharmacol., 98 (2015) 1-15. [3] D. Pettersen, O. Davidsson, C. Whatling, Recent advances for FLAP inhibitors, Bioorg. Med. Chem. Lett., 25 (2015) 2607-2612. [4] S.C. Lazarus, T. Lee, et al., Safety and clinical efficacy of zileuton in patients with chronic asthma, Am. J. Manag. Care, 4 (1998) 841-848. [5] M. Lemurell, J. Ulander, S. et al., Discovery of AZD6642, an inhibitor of 5-lipoxygenase activating protein (FLAP) for the treatment of inflammatory diseases, J. Med. Chem., 58 (2015) 897-911. [6] N.S. Stock, G. Bain, et al., 5-Lipoxygenase-activating protein (FLAP) inhibitors. Part 4: development of 3-[3-tert-butylsulfanyl-1-[4-(6-ethoxypyridin-3-yl)benzyl]-5-(5-methylpyridin-2-y lmethoxy)-1H-indol-2-yl]-2,2-dimethylpropionic acid (AM803), a potent, oral, once daily FLAP inhibitor, J. Med. Chem., 54 (2011) 8013-8029. [7] H. Takahashi, D. Riether, et al., Synthesis, SAR, and series evolution of novel oxadiazole-containing 5-lipoxygenase activating protein inhibitors: discovery of 2-[4-(3-{(r)-1-[4-(2-amino-pyrimidin-5-yl)-phenyl]-1-cyclopropyl-ethyl}-[1,2,4]ox adiazol-5-yl)-pyrazol-1-yl]-N,N-dimethyl-acetamide (BI 665915), J. Med. Chem., 58 (2015) 1669-1690. [8] C. Whatling, W. McPheat, M. Herslof, The potential link between atherosclerosis and the 5-lipoxygenase pathway: investigational agents with new implications for the cardiovascular field, Expert Opin Investig Drugs, 16 (2007) 1879-1893. [9] E. Banoglu, B. Caliskan, et al., Identification of novel benzimidazole derivatives as inhibitors of leukotriene biosynthesis by virtual screening targeting 5-lipoxygenase-activating protein (FLAP), Bioorg. Med. Chem., 20 (2012) 3728-3741. [10] C. Pergola, J. Gerstmeier, et al., The novel benzimidazole derivative BRP-7 inhibits leukotriene biosynthesis in vitro and in vivo by targeting 5-lipoxygenase-activating protein (FLAP), Br. J. Pharmacol., 171 (2014) 3051-3064. [11] E. Banoglu, E. Celikoglu, et al., 4,5-Diarylisoxazol-3-carboxylic acids: A new class of leukotriene biosynthesis inhibitors potentially targeting 5-lipoxygenase-activating protein (FLAP), Eur. j. Med. Chem., 113 (2016) 1-10. [12] P.S. Mundi, J. Sachdev, C. McCourt, K. Kalinsky, AKT in Cancer: New Molecular Insights and Advances in Drug Development, Br J Clin Pharmacol, (2016).